Apparatus
Major Breakthrough In 27 Years! This Cancer Vaccine Has Kept Deadly Brain Cancer Patients Alive For Over 8 Years!
A dendritic cell vaccine has shown excellent results in the treatment of glioblastoma, significantly extending the survival of patients with a good safety profile, according to new data from a global clinical trial. To date, patients who have benefited for the longest time have survived for more than eight years.

A global clinical trial has released new data on a dendritic cell vaccine that has shown excellent results in treating glioblastoma, significantly extending the survival of patients and having a good safety profile. To date, patients who have benefited for the longest time have survived for more than eight years.

A global clinical trial led by King's College Hospital has recently published the results of a Phase 3 clinical trial (NCT00045968) in which a dendritic cell vaccine called DCVax-L, in combination with standard treatment, significantly extended the overall survival of patients with newly diagnosed glioblastoma and patients with recurrent glioblastoma.
This cancer vaccine not only extends the survival of patients by months, but in some cases, even years, with the longest beneficiary assemble having survived for over 8 years to date.
This is the first time in 17 years that such dramatic results have been achieved in the systemic treatment of newly diagnosed glioblastoma patients and the first time in 27 years that a treatment has been shown to extend survival in patients with recurrent glioblastoma.
The results of the trial were published in JAMA Oncology on November 17, 2022.
Trial details
The trial spanned more than eight years and researchers recruited 331 patients with newly diagnosed glioblastoma from the UK, US, Canada and Germany from August 2007 to November 2015. The mean age of the patients was 56 years (range 19-73 years). The majority of participants (61.0%) were male and 88.8% of patients were white. 91.5% of patients were enrolled from 2012 to 2015.
All patients received surgical resection, recovery, leukapheresis and 6 weeks of standard post-operative radiotherapy care prior to enrolment. The mean time from surgery to randomisation to the group was 3.1 months.
Following initial diagnosis, all patients underwent collection of tumour tissue to create the DCVax-L vaccine. Patients received DCVax-L or placebo on days 0, 10 and 20, followed by monthly temozolomide as standard of care at months 2, 4 and 8 and months 12, 18, 24 and 30.
Of the 331 patients enrolled, the researchers randomly assigned 232 patients to the initial DCVax-L treatment group and 99 patients to the placebo group.
After tumour recurrence, 64 of the 99 patients in the placebo group crossed over to DCVax-L. 18 of the 64 patients (28.1%) were transferred to the DCVax-L group after disease progression and received treatment following resection of the original tumour.
Trial data results
Among patients with newly diagnosed glioblastoma, the mean overall survival was 19.3 months for patients in the DCVax-L treatment group and 16.5 months for the control group; survival rates were 15.7% and 9.9% at 48 months and 13.0% and 5.7% at 60 months for the two groups, respectively.
The researchers concluded that DCVax-L resulted in a 20% relative reduction in the risk of death at any time point in patients with newly diagnosed glioblastoma. Furthermore, this relative survival benefit increases over time.
Thirteen per cent of patients treated with DCVax-L survived for at least five years from their glioblastoma diagnosis, compared to 5.7 per cent of controls, and patients assemble for longer than eight years.
In patients with newly diagnosed methylated MGMT, the mean overall survival of patients in the DCVax-L treatment group was 30.2 months compared to 21.3 months in the control group.
In patients with recurrent glioblastoma, the mean overall survival of patients in the DCVax-L treatment group was 13.2 months, compared to 7.8 months in the control group.
The researchers concluded that DCVax-L treatment in patients with recurrent glioblastoma at the time of their first recurrence resulted in a 42% relative reduction in the risk of death at any time point. Again, this relative survival benefit increases over time.
At 24 months after relapse, the overall survival rate for patients in the DCVax-L treatment group was 20.7% compared to 9.6% for the control group. At 30 months, the overall survival rate for patients in the DCVax-L treatment group was 11.1% compared to 5.1%% for the control group.
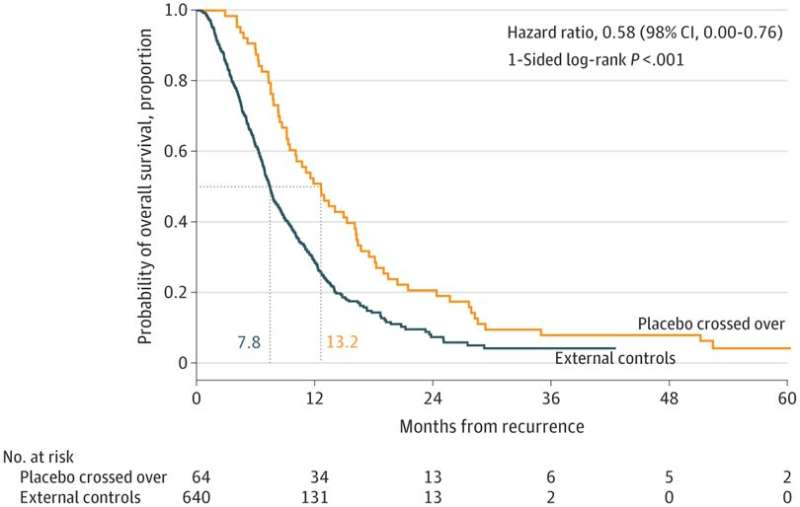
Kaplan-Meier (survival curve) plot of overall survival of patients with recurrent glioblastoma
Northwest Biotherapeutics, the company that manufactures the DCVax-L vaccine, said this is the first Phase 3 clinical trial result in nearly 20 years that has been shown to significantly extend survival in patients with glioblastoma and the first Phase 3 clinical trial result in nearly 30 years that has been shown to result in a survival benefit for patients with recurrent glioblastoma.
"We are pleased to see significantly longer survival for glioblastoma patients treated with DCVax-L in this trial, with patient survival rates more than twice that of the existing standard of care." Linda F. Powers, Chief Executive Officer of Northwest Biotherapeutics, said in a press release. "With more than 400 failed glioblastoma clinical trials over the past 15 years, it is very gratifying to be able to offer new hope to patients facing this devastating disease."
Safety
Safety-wise, DCVax-L treatment was well tolerated. During the course of the trial, there were only five serious adverse events that could be associated with the investigational treatment out of the 2151 total doses given to patients. There was no evidence of any autoimmune reactions or cytokine storms in patients in the experimental group.
Linda F. Powers added, "It is particularly encouraging to see these survival extension data and such a benign, safe treatment. With over 2,100 doses of DCVax-L administered during the trial, we found no significant differences in the adverse event profile from standard treatment alone."
About Glioblastoma
Glioblastoma is one of the more aggressive primary malignant brain tumours and is extremely difficult to treat as its pathogenesis is one of the more complex of brain tumours. The 5-year survival rate for patients with glioblastoma is only about 5%.
At the same time, glioblastoma is highly susceptible to recurrence as it invades normal brain tissue and is difficult to completely remove through surgery.
Immunosuppressive cells in the brain tumour microenvironment prevent immunotherapy from working, while the presence of the blood-brain barrier in the brain prevents therapeutic drugs from entering the brain, limiting the use of conventional and novel therapies. As a result, breakthroughs in the treatment of glioblastoma have long been elusive.
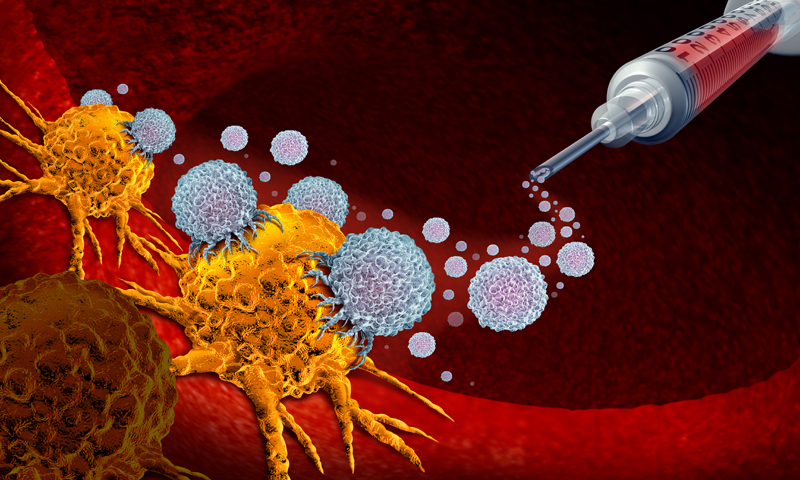
About the DCVax-L vaccine
DCVax-L is a dendritic cell vaccine designed to be developed individually for each patient by isolating specific immune cells (dendritic cells) from the patient's blood. These cells are then activated with biomarkers from the patient's tumour sample. When the vaccine containing the cells is injected back into the patient, it helps the patient's entire body's immune system to recognise and attack the cancer cells.
DCVax-L is a fully personalised immunotherapy made from the patient's own dendritic cells and antigens collected from the patient's own tumour sample. The researchers produce a set of doses from a single manufacturing batch that can be used for many years, and then store the product frozen in individual doses for 'off-the-shelf' use by patients throughout their treatment regimen.
Unlike traditional anti-cancer drugs, DCVax-L is unique in that it does not target a single antigen, but rather uses multiple active ingredients to target multiple targets in the cancer and to maximise inhibition of tumour cell escape.
Patient case study

A patient at King's College Hospital, Nigel French, 53, was diagnosed with glioblastoma in 2015 after suffering from nocturnal seizures and subsequently underwent surgery at King's College Hospital. On the advice of his doctor, Nigel French was enrolled in the DCVax-L trial.
Now, seven years after receiving the DCVax-L vaccine, Nigel French is still in remission and is living a good life.
Nigel French says he is grateful to have taken part in the trial and tried the DCVax-L vaccine. "I am very grateful to the team at King's College Hospital for providing me with this lifeline and the DCVax-L treatment combined with a positive mindset has given me my life back."
Professor Keyoumars Ashkan, Professor of Neurosurgery at King's College Hospital and European Principal Investigator of the clinical trial, said, "Immunotherapy is a very promising approach to cancer treatment and the assorted results of this phase 3 trial have now been revealed and published, offering new hope to patients with glioblastoma.
The DCVax-L vaccine has been shown to extend the lives of patients with glioblastoma, including those who were previously considered to have a poorer prognosis, such as the elderly patient group, and those who cannot undergo radical surgery for technical or other reasons, who benefit more significantly."

-
![]()
![]() ApparatusFeb 22, 2026
ApparatusFeb 22, 2026New CAR-T Therapy CB-010, Granted Two FDA Designations And Three Patients Are Cancer-Free For Six Months!
-
![]()
![]() ApparatusFeb 21, 2026
ApparatusFeb 21, 2026Mysterious gel quickly seals wounds
-
![]()
![]() ApparatusFeb 20, 2026
ApparatusFeb 20, 2026Fujifilm Ai Technology Can Predict Whether Cognitive Disorders Will Progress To Alzheimer's Disease
-
![]()
![]() ApparatusFeb 19, 2026
ApparatusFeb 19, 2026Stavudol Paediatric Indication Approved For The Treatment Of Children Aged 3 Months And Older With Complicated Intra-Abdominal Infections
-
![]()
![]() ApparatusFeb 18, 2026
ApparatusFeb 18, 2026New Drug For IDH1 Mutation/Relapsed/Refractory Acute Myeloid Leukaemia With Good Complete Remission Rates And Manageable Toxicity