Apparatus
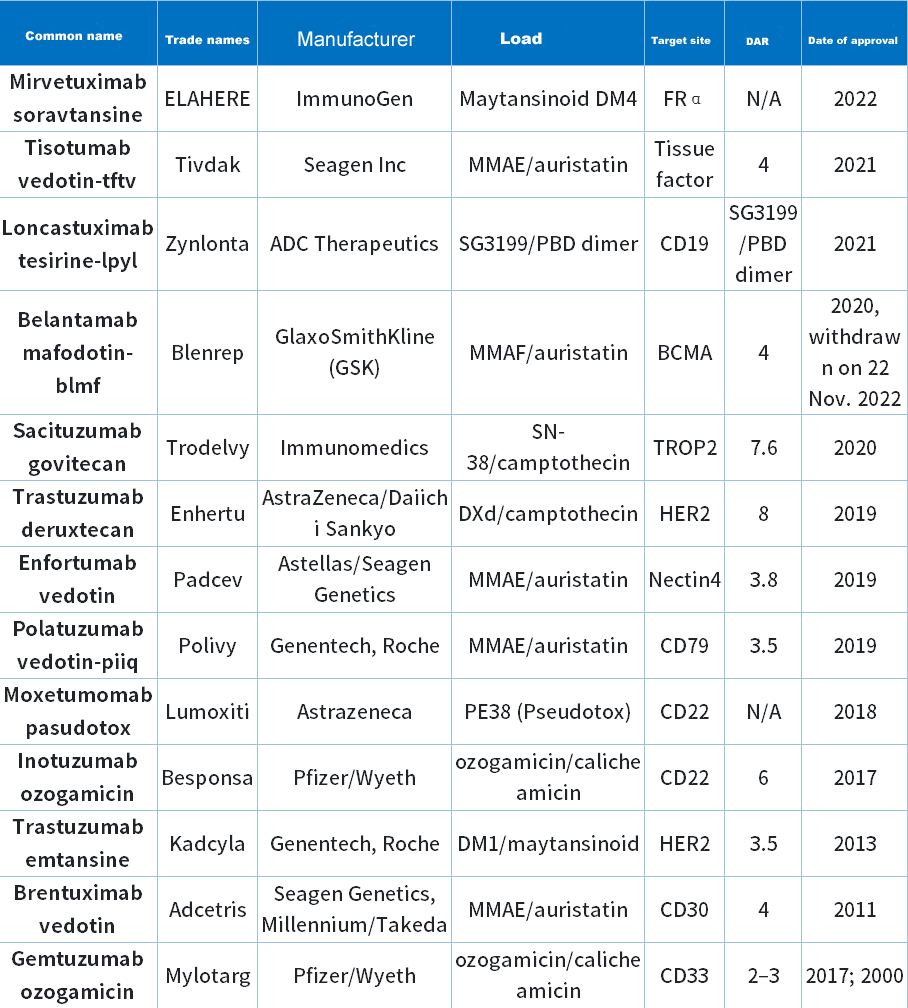
Inventory Of Fda-Approved Adc Drugs
The global ADC drug market will be approximately $5.2 billion by 2021
Antibody-drug conjugates (ADCs) are used to link antibodies to small molecule cytotoxic drugs using a specific linker, the main components of which include the antibody, the linker and the small molecule cytotoxic drug.
Antibody molecules primarily play a role in targeting delivery, while small molecule drugs play a role in exerting therapeutic effects.
However, some antibodies also have an anti-tumour effect.
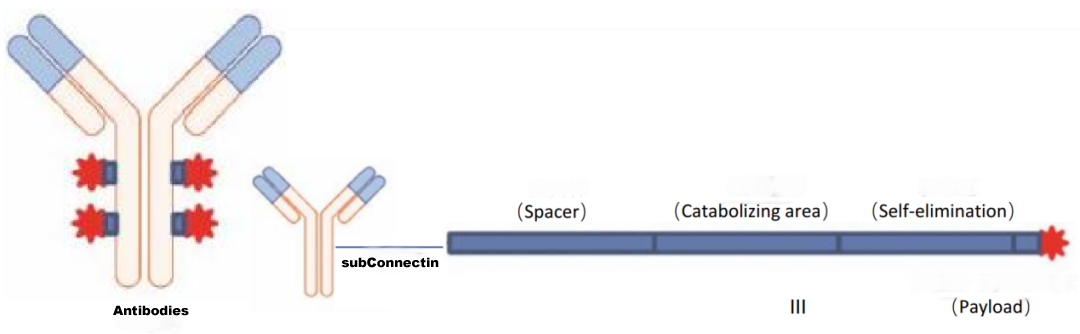
Figure 1 ADC drug structure
Compared to other chemotherapeutic drugs, ADC drugs greatly enhance the specificity of drug delivery through the specific binding of antigen and antibody.
The antibody binds to the specific antigen on the tumour cell membrane and induces endocytosis, allowing the antibody and the cytotoxic small molecule drug attached to it to enter the cell, where it is then degraded by lysosomes.
The small molecule drug is released into the cell and induces apoptosis through DNA insertion or inhibition of microtubule synthesis.
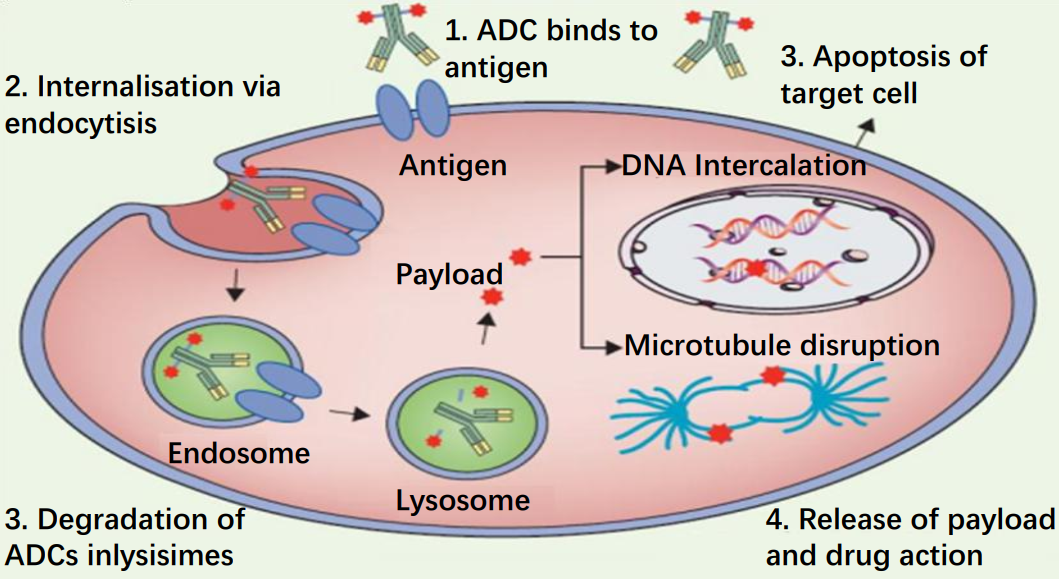
Figure 2 Mechanism of action of ADC drugs
As the most popular research topic, ADC technology aims to target and kill only cancer cells without harming healthy cells. After more than 10 years of development, there are currently 13 ADC drugs approved by the FDA (Table 1).
Table 1 FDA-approved ADC drugs
2022
FDA approves only one ADC drug
In 2022, the FDA approved only 1 ADC drug, ELAHERE (Mirvetuximab soravtansine-gynx).
It is the world's first ADC drug for the treatment of platinum-resistant ovarian cancer, developed by the US partner ImmunoGen, Inc. of China East China Pharmaceutical Co.

Figure 4 Accelerated FDA approval for ELAHERE
The primary basis for the FDA's accelerated approval of Elahere was Study 0417 (NCT04296890). This was a single-arm trial with 106 patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancers. Patients were allowed to receive up to three lines of systemic therapy. All patients are required to receive bevacizumab.
The trial recruits patients whose tumours have been determined to be positive for FRα expression. Patients receive ELAHERE 6 mg/kg (based on adjusted ideal body weight) intravenously every three weeks until disease progression or unacceptable toxicity. Tumour response assessments were performed every 6 weeks for the first 36 weeks and every 12 weeks thereafter.
The results of the study showed a confirmed overall response rate (ORR) of 31.7% (95% CI: 22.9,41.6) and a median duration of response (DOR) of 6.9 months (95% CI: 5.6,9.7) in an evaluable efficacy population (104 patients) resistant to platinum-based agents, with measurable disease and receiving at least one treatment.
The most common (≥20%) adverse reactions to ELAHERE, included visual impairment, fatigue, increased aspartate aminotransferase, nausea, increased alanine aminotransferase, keratoconus, abdominal pain, lymphopenia, peripheral neuropathy, diarrhoea, reduced albumin, constipation, increased alkaline phosphatase, dry eye, reduced magnesium, leukopenia, neutropenia and haemoglobin reduction.
Domestic ADC drug development
In 2021, the global ADC drug market will be approximately US$5.2 billion; since the approval of the first ADC product, emtricitumomab, in January 2020, 11 indications for five ADC products have been approved in more than two years.
There are also multiple target ADC candidates currently in Phase III, with a rich overall pipeline.
The ADC drug industry has seen significant clustering of targets and indications, with significant competition.
In terms of indications, the main clustering is in the field of oncology, with approximately 95.2% (nearly 500 products) of the pipeline layout indications being oncology, followed by autoimmune diseases accounting for approximately 10 projects in preclinical.
In terms of targets, the main focus is on HER2 targets, accounting for about 38.4%, with a cumulative total of about 60 product projects; EGFR targets account for about 11.9%, with a cumulative total of about 20 product projects; the rest of the targets do not exceed 10%.
Difficulties in ADC drug development
It has been more than 100 years since the concept of ADC was introduced, but there are still very few varieties on the market, and most of those under investigation are still in the early stages.
The main reason for this is the difficulty of developing ADC drugs and the high technical barriers. ADC drugs need to go through multiple steps to work once they enter the human body, and each step has technical difficulties to overcome.
Despite the increasing number of approved ADCs, challenges remain for ADCs that have demonstrated superior safety and efficacy in the clinic.
An unexpected problem faced by many developers during the clinical evaluation process is the inability of ADCs to demonstrate benefit compared to controls, such as Rovalpitumab tesirine (RovA-T), for which the Phase I trial reported an 18% ORR in evaluable patients and a 38% ORR in patients with high DLL3 expression.

However, the trial did not meet the primary endpoint and reported high rates of toxicity due to the results of the Phase II trial TRINITY (NCT02674568) which raised safety and efficacy issues. The most common event in patients was pleural effusion, which was considered to be a PBDE dimer-related toxicity. Ultimately, the results of the Phase 3 trials TAHOE (NCT03061812) and MERU (NCT03033511) led AbbVie to stop development of RovA-T altogether due to the lack of survival benefit compared to the control arm.
Another challenge for ADC is off-target toxicity, which is caused by the premature release of cytotoxic small molecules into the bloodstream. Antibodies are clumsy transporters and have difficulty reaching tumour tissue, with only an estimated 0.1% of the drug reaching tumour tissue. To ensure that the other 99% of highly toxic warheads do not cause systemic toxicity, the chemical link between warheads and antibodies must be sufficiently stable. However, in order to release the warhead in the cell without being very stable, this obviously poses a problem for drug design.
In addition, ADCs are prone to aggregation. the aggregation of ADCs leads to modifications that reduce their ability to bind antigens. Protein aggregation is a major obstacle to ADC development. It can occur at every stage as well as during transport and long-term storage. Aggregation is immunogenic. In addition, protein aggregation may lead to product loss. In general, any chemical or physical degradation can lead to structural changes in ADCs and result in excessive protein aggregation.

In addition to the problems mentioned above, ADC drugs also face problems of resistance and immunogenicity.
Despite the many challenges, this is an era of innovative drugs, with newer iterations of different technologies and the need for broader clinical coverage and validation of ADCs as more issues are investigated in depth.
In the future, ADC drugs will have great potential in the antineoplastic market.
-
![]()
![]() ApparatusFeb 27, 2026
ApparatusFeb 27, 2026Clinical Approval For Colombotide Skb264 (Mk-2870) In Combination With Oxitinib
-
![]()
![]() ApparatusFeb 26, 2026
ApparatusFeb 26, 2026New Drug For IDH1 Mutation/Relapsed/Refractory Acute Myeloid Leukaemia With Good Complete Remission Rates And Manageable Toxicity
-
![]()
![]() ApparatusFeb 25, 2026
ApparatusFeb 25, 2026J. Enzyme Inhib. Med. Chem. | Protac Targeting Aimp2-Dx2: a New Therapy For Lung Cancer
-
![]()
![]() ApparatusFeb 24, 2026
ApparatusFeb 24, 2026Fda Accepts Zuranolone's Nda Application And Grants It Priority Review For The Treatment Of Mdd And Pdd
-
![]()
![]() ApparatusFeb 23, 2026
ApparatusFeb 23, 2026Georgia Institute of Technology 3D printing heart valve model to improve the success rate of valve replacement